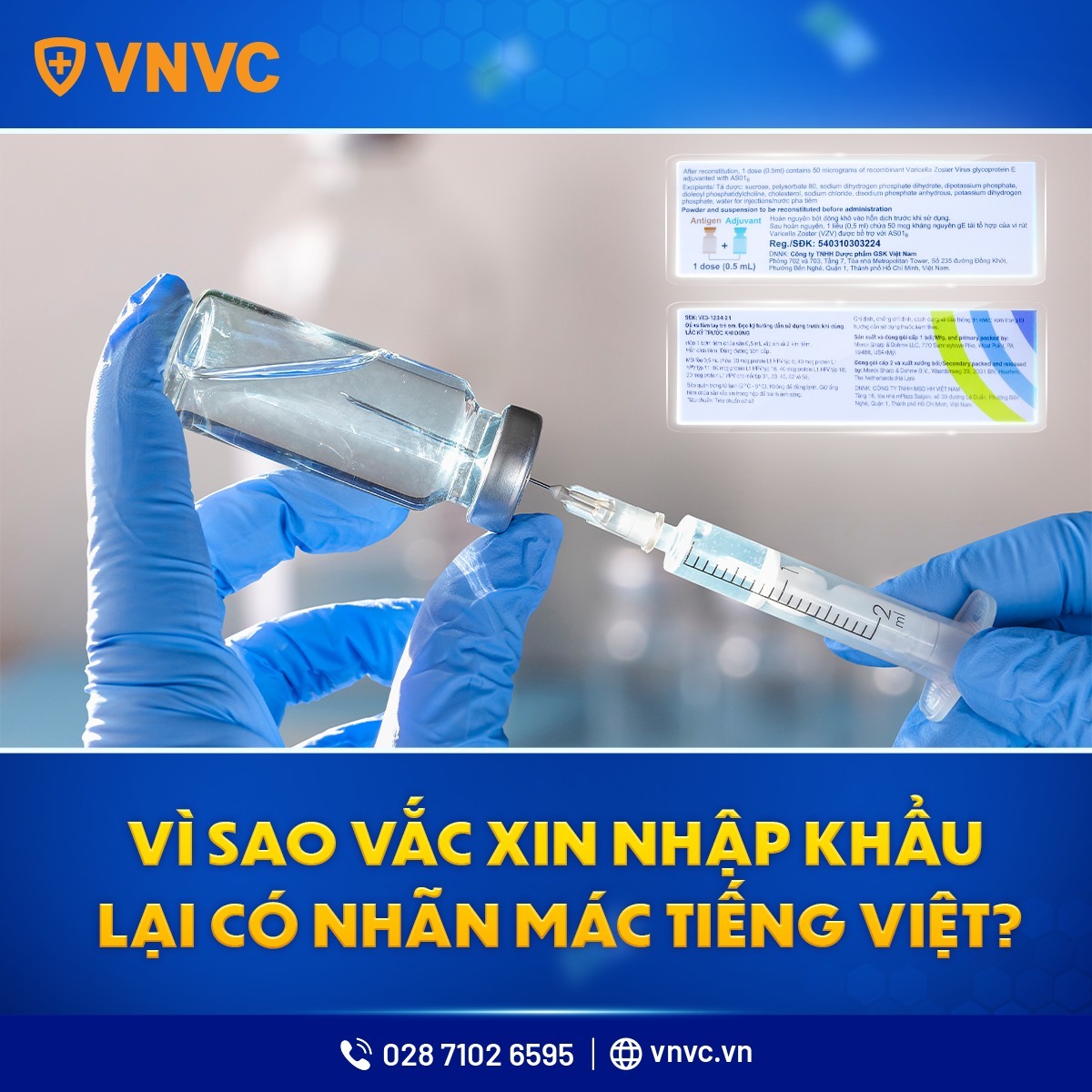
🔥 VNVC – Why Do Imported Vaccines Have Vietnamese Labels?
Many customers wonder why some imported vaccines have labels or instructions in Vietnamese. VNVC provides the following explanation:
✅ According to the Ministry of Health regulations:
Vietnamese labeling is mandatory: According to Circular 01/2018/TT-BYT, all imported medicines/vaccines must have labels and instructions for use in Vietnamese.
Supplementary labels: If the original label lacks required information, the importer must add a supplementary Vietnamese label at a GSP-compliant warehouse before distribution.
Proactive action by manufacturers: Some manufacturers voluntarily print Vietnamese labels and instructions at the factory to streamline the process.
✅ VNVC’s commitment:
All vaccines are genuine, with clear origins, sourced from reputable companies like Pfizer, GSK, Sanofi, AstraZeneca, etc.
The processes of importation, storage, transportation, and usage all comply with GSP, GDP standards and WHO guidelines.
A highly qualified medical team ensures safe vaccination procedures and strict post-vaccination health monitoring.
➡️ The presence of Vietnamese labels on imported vaccines complies with regulations and helps the public access product information transparently and accurately.